Eventually your SMA patient may not be able to scroll down this website.
Explore the site to know more.*
how would your patients cope with losing
basic motor functions?
don't wait to find out - refer them
to a specialist neurologis today
Without intervention, people living with spinal muscular atrophy (SMA) will continue to experience disease progression.1 The ability to stabilise patients’ disease is considered an important goal as it allows valuable motor functions and quality of life to be preserved.
Explore the sections below to learn more about what matters most to patients, as well as the latest updates to standards of care that make referral to an SMA specialist your crucial next step.
RECOGNISING SMA
RECOGNISING SIGNS AND SYMPTOMS OF SMA IN ADULTS
Less severe forms of SMA can emerge and be diagnosed in adulthood.2,3 Compared to childhood SMA, adult SMA may have milder symptoms, but is still progressive. Compared to SMA in childhood, the course of SMA in adults can be more insidious and difficult to recognise.4
This is why it is crucial to know how to identify the main signs and symptoms: if you suspect SMA in one of your patients, refer them to a specialist.
In the adult patient, a detailed history may help point to a diagnosis, including:
- Pattern of progression of symptoms
- Age at onset of symptoms
- Age at which bracing was provided to maintain ambulation or wheelchair was required (if applicable)
- Current and past ambulatory distances
In the image below, you can explore the signs and symptoms that are typically appearing in people living with SMA.
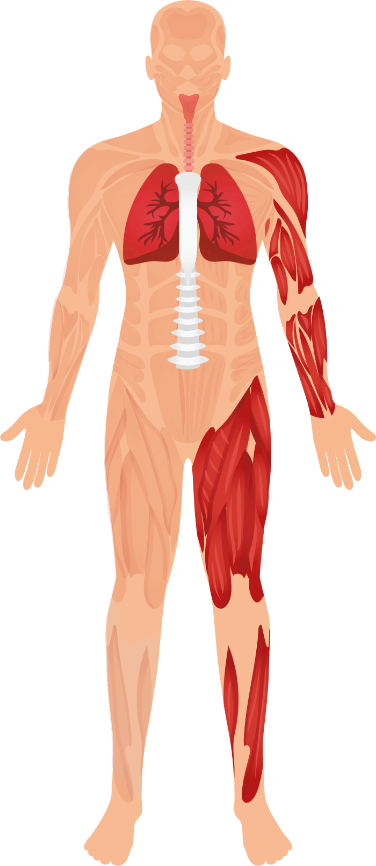
BREATHING ABILITY
- e.g. difficulty in raising the arms above the head
- especially in hips & shoulders
- e.g. difficulty running or climbing stairs
This is the checklist of the signs and symptoms
organised around the domain they affect:
CARDINAL MOTOR SYMPTOMS2,5
BONE SYMPTOMS2,5
RESPIRATORY SYMPTOMS2,6
GENERAL SYMPTOMS2,6
A PATIENT’S PERSPECTIVE:
“I’m Normal. Just Different”
SAMUEL - A young adult living with SMA, who was diagnosed as an adolescent and his perspective on life with SMA.
Watch video nowvideoWrapperReversedSamuel
HELP YOUR PATIENTS HOLD THEIR
INDEPENDENCE.
refer theM to a specialist
neurologist today
DISEASE IMPACT - HOW ARE LIVES BEING AFFECTED?
LIVES COME TO A STANDSTILL WITH SMA
SMA is a debilitating neuromuscular disease characterised by motor neuron degeneration and loss of muscle strength.7 As the disease progresses, patients face the gradual erosion of pulmonary and motor functions to the point where even simple tasks become impossible.1,8-10
Given that prospect, any treatment that can stabilise disease progression will be welcomed as a therapeutic breakthrough.10
HELP YOUR PATIENTS LIVING WITH SMA PROTECT
WHAT THEY CHERISH MOST.
REFER THEM TO A SPECIALIST
NEUROLOGIST TODAY
PATIENTS’ EXPECTATIONS
THE IMPORTANCE OF STABILISING SMA IN PATIENTS
People living with SMA have become aware that disease stabilisation is key to maintaining an optimal quality of life.10
Optimising the care of adult patients living with SMA is all about delivering high-quality, evidence-based, and patient-centered healthcare.11 More importantly, it’s about helping patients get the most from their lives on a day-to-day basis.
Why is disease stabilisation
IMPORTANT?
The results of a European-wide survey** of patients and their caregivers identified the top three functions that patients hoped to preserve in order to maintain their independence:10
TOP 3
functions patients want to PRESERVE are:
81%
of PARTICIPANTS
felt that a treatment that could stabilise their disease would represent MAJOR THERAPEUTIC PROGRESS10
Disease stabilisation is a relevant therapeutic outcome in adults with SMA.12
Things that people often take for granted like being able to shower or bathe, using the toilet, and being able to eat independently are essential for everybody but especially for those people living with SMA, because it means sustaining independence and dignity.10
VOICES FROM PEOPLE LIVING WITH SMA
Once thought to be primarily a childhood disease, SMA can be diagnosed in adolescents and adults.13 Meet Michel and Samuel and put yourself in the shoes of adults living with SMA or their family.
A CAREGIVER’S PERSPECTIVE:
“I wish I had pushed harder”
MICHEL - A caregiver of a young adult living with SMA and his family’s journey.
Signs and SymptomsvideoWrapper2
Treatment expectations for adults living with SMA have changed.
Download the step-by-step guide below or visit the patient site to gain tips about discussing potential care options.
**A questionnaire assessing the impact of Type 2 or Type 3 SMA on patients’ quality of life and their expectations relating to new treatments was sent out directly via electronic mailing to the targeted patient population by SMA-Europe member organizations in July 2015. 822 valid replies were collected from 16 countries.10
HFMSE=Hammersmith Functional Motor Scale-Expanded
STANDARDS OF CARE - WHAT HAS CHANGED?
A LOT HAS CHANGED IN THE QUALITY OF CARE FOR PEOPLE LIVING WITH SMA
People living with SMA can expect better outcomes through better care options. Improved management of the disease coupled with different care options could lead to a better quality of life for people living with SMA.14,15
Ongoing research is providing an ever-increasing understanding of the science behind SMA and care options.14-16
There has been considerable research into SMA in recent years:3,17-19
INCREASE
in SMA studies between 2001-2011 and 2011-2021*17,18
CLINICAL TRIALS
currently studying SMA*19
Most importantly, specialist neurologists now have access to care options with the potential to stabilise your patient’s disease and help preserve the functions so important to them.14,15,20
* Calculated based on search results on data accurate as of 22 March 2021
If you are interested in learning more about Biogen's offering
HAVE YOU SEEN THE UPDATED STANDARDS OF CARE FOR SMA?
- In 2007, an International Conference on the Standards of Care for SMA published a consensus statement on SMA standards of care that has been widely used throughout the world.4
- In 2018, a two-part update of these earlier recommendations was published, which includes changes to the management and care of patients with SMA, along with information around new care options.14,15
Updates on diagnosis, rehabilitation, orthopaedic and spinal management; and nutritional, swallowing and gastrointestinal management:14
Updates on pulmonary management, acute care, other organ involvement, ethical issues, medications, and the impact of new care options:15
TOOLS THAT CAN HELP
To help facilitate discussions with your patient, there are patient-support materials you can download:
Summary of the latest SMA Standards of Care for SITTERS
Summary of the latest SMA Standards of Care for WALKERS
be sure that your patient is receiving the
most up-to-date standards of care.
refer them to a specialist
neurologist today
ACT NOW TO STABILISE SMA
YOU CAN MAKE A DIFFERENCE
It is important that you talk with your patient about the benefits of a specialist neurologist referral.
Without intervention, SMA patients will continuously lose motor function.1 Acting early enough can make a difference to the life of your SMA patient.
STARTING WITH GENETIC TESTING
It is important that your patients are genetically tested.
Once referred, a specialist neurologist will provide your patient with a genetic test (if they haven’t had one already).
Genetic testing will identify whether your patient is eligible for care options that are suitable for those with a specific genetic diagnosis.1
If your patient has a condition that is not SMA, referral will allow other care options to be discussed.21
Treatment options for adults living with SMA have changed
Download the HCP-Patient Dialogue Booklet to find out more about the benefits of treatment, shared-decision making and what is important for adults living with SMA.
You can also suggest to your patients to visit the patient site for more information.
HELP YOUR SMA PATIENTS HOLD ON
TO THEIR INDEPENDENCE.
REFER THEM TO A SPECIALIST
NEUROLOGIST TODAY
The characters shown are real patients and the required consent to use their stories has been obtained from the patients and families. Photographs are for illustrative purposes only.
FIND AN SMA SPECIALIST NEUROLOGIST USING THE LOCATOR BELOW
If you want further information on SMA
*Potential ability to scroll down a website is supported by: Item C: Tracing a Path and Item G: Pushing a Button Light on the Revised upper limb module for spinal muscular atrophy22
References
1. Kaufmann P, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurol 2012;79:1889-1897.
2. Montes J. Spinal Muscular Atrophy in Adults. [online] [cited 2020 Nov 30]. Available from: URL:
https://www.neuropt.org/docs/degenerative-diseases-sig/spinal-muscular-atrophy-in-adults.pdf?sfvrsn=8d2aae96_2.
3. Juntas Morales R, Pageot N, Taieb G, Camu W. Adult-onset spinal muscular atrophy : and update. Rev Neurol (Paris) 2017;173(5):308-319.
4. Wang CH, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol 2007;22(8):1027-1049.
5. Arnold WD, et al. Muscle Nerve. 2015;51(2):157-67.
6. Wijngaarde CA, et al. Orphanet J Rare Dis. 2020;15(1):88.
7. Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 2008;371:2120-2133.
8. Wadman RI, Wijngaarde CA, Stam M, et al. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with SMA types 1c-4. Eur J Neurol 2018;25(3):512-518
9. Paradis AD, Wassel CL, Dreyfus J, et al. Symptoms and Complications Among Later Childhood, Adolescent, and Adult Spinal Muscular Atrophy Patients: A Natural History Study Within US Hospitals. Poster P206 presented at MDA 2019; Orlando, Florida, USA.
10. Rouault F, et al. Disease impact on general well-being and therapeutic expectations of European Type II and Type III spinal muscular atrophy patients. Neuromuscul Disord 2017;27(5):428-438.
11. Wan HWY, et al. “Getting ready for the adult world”: how adults with spinal muscular atrophy perceive and experience healthcare, transition and well-being. Orphanet J Rare Dis 2019;14:74.
12. Gusset N, et al. Understanding European patient expectations towards current therapeutic development in spinal muscular atrophy. Neuromuscular Disorders 2021 Feb 03. doi:https://doi.org/10.1016/j.nmd.2021.01.012.
13. NIH. Spinal Muscular Atrophy Factsheet: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Spinal-Muscular-Atrophy-Fact-Sheet.
14. Mercuri E, et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscl Disord 2018;28(2):103-115.
15. Finkel R, et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord 2018;28(3):197-207.
16. Bharucha-Goebel D, Kaufmann P. Treatment Advances in Spinal Muscular Atrophy. Curr Neurol Neurosci Rep 2017;17(11):91.
17. National Center for Biotechnology Information. Spinal muscular atrophy. Pubmed website. [online] [cited 2021 Mar 22] Available from: URL: https://pubmed.ncbi.nlm.nih.gov/?term=%28spinal+muscular+atrophy%29+AND+%28%28%222018%2F01%2F01%22%5BDate+- +Publication%5D+%3A+%222018%2F12%2F31%22%5BDate+-+Publication%5D%29%29&sort=.
18. National Center for Biotechnology Information. Spinal muscular atrophy. PubMed website. [online] [cited 2021 Mar 22] Available from: URL: https://pubmed.ncbi.nlm.nih.gov/?term=%28spinal+muscular+atrophy%29+AND+%28%28%222010%2F01%2F01%22%5BDate+- +Publication%5D+%3A+%222010%2F12%2F31%22%5BDate+-+Publication%5D%29%29&sort=.
19. Search for ‘Recruiting, Active, not recruiting. Enrolling by invitation Studies. Spinal Muscular Atrophy. ClinicalTrials.gov. [online] [cited 2021 Mar 22] Available from: URL: https://www.clinicaltrials.gov/ct2/results?cond=%22Spinal+muscular+atrophy%22&term= &type=&rslt=&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city =&dist=&locn=&rsub=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&rfpd_s=&rfpd_e=&lupd_s=&lupd_e=&sort=.
20. Gusset N, et al. Understanding European patient expectations towards current therapeutic development in spinal muscular atrophy. Neuromuscular Disorders 2021 Feb 03. doi: https://doi.org/10.1016/j.nmd.2021.01.012.
21. Visser J, et al. Mimic syndromes in sporadic cases of progressive spinal muscular atrophy. Neurology 2002;58(11):1593-1596.
22. Mazzone ES, et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve. 2017 Jun;55(6):869-874. doi: 10.1002/mus.25430. Epub 2017 Feb 6.























